Succeeding in the DiGa landscape

The transformation towards digital healthcare is progressing rapidly today in order to offer insured individuals the broadest possible range of care and to make processes more efficient. Digital health applications (DiGA for short) are playing an increasingly important role in the context of physician shortages and significantly increased healthcare costs for those with statutory health insurance. In this article, we will examine how the DiGA landscape has developed in Germany and its direct neighbors in recent years, and whether DiGA have been able to establish themselves within the framework of integrated care. Furthermore, we will focus on exemplary factors that are decisive for sustainable success in the market. Finally, we would also like to provide a brief insight into the further development potential of digital health applications.
The Digital Health Application
As part of the ongoing digitization of the healthcare system, digital health applications (DiGA for short) have become increasingly widespread in Germany since 2019. These "digital helpers" are intended to support patients in addition to medical/therapeutic treatment based on digital technologies in order to fulfill a specific medical purpose through their corresponding main function. The primary function of such digital applications includes areas ranging from the detection or monitoring of diseases to the alleviation or compensation of disease- or disability-associated symptoms.
Such support can then be billed to health insurance companies for those with statutory health insurance in accordance with the Digital Healthcare Act (DVG). In order to be reimbursable, a listing in the so-called DiGA directory is necessary after evaluation by the Federal Institute for Drugs and Medical Devices (BfArM) in accordance with § 139e SGB V [1, 2].
In the first part of this white paper series, we have summarized when a listing would be useful for you as a medical device manufacturer, what the process of such a listing is (keyword "fast-track procedure"), and what basic factors you need to consider.
Status quo
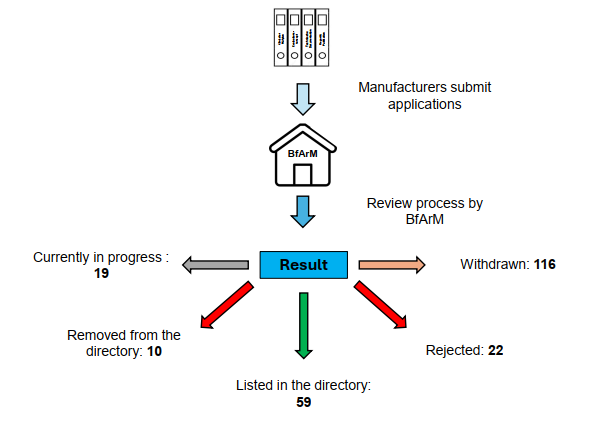
The DiGA landscape has been steadily growing in recent years. Currently, there are 226 applications for listing with the BfArM, which have been submitted via the DiGA application portal. Of these, 177 applications have been submitted for provisional inclusion and testing, and 49 applications have been submitted for permanent inclusion (BfArM, as of March 12, 2025).
However, it should be mentioned that, in retrospect, only a certain proportion of the submitted applications were successful, with manufacturers tending to withdraw their application for listing rather than the submitted application being actively rejected by the BfArM. This may be related to the fact that after a negative decision, a waiting period of 12 months applies before a new application for inclusion can be submitted [3].
DiGA Landscape Germany – Current Figures and Facts
In recent years, the number of digital health applications included in the DiGA directory has steadily increased. Currently, a total of 69 DiGA are listed in the DiGA directory. 19 DiGA were initially included provisionally and 40 permanently. Of the listed DiGA, regardless of whether provisional or permanent, 42% are in the category of psyche (including support for depression, panic disorder/anxiety disorders or psychotic symptoms), followed by 14.5% in the area of support for muscles, bones and joints. DiGA on the subject of hormones and metabolism accounted for 13%.
In addition to the included digital health applications, 10 were also removed from the listing, which corresponds to approximately 14.5%. The reasons for removal from the DiGA directory were as follows:
- The DiGA was removed from the DiGA directory at the request of the manufacturer (4).
- A positive care effect could not be demonstrated for the DiGA (3).
- The trial study for the DiGA in question could not be completed within the trial period. Accordingly, no positive care effect was demonstrated during the trial period (1).
- No exact information available (2).
According to the change history, 4 DiGA were permanently listed in the directory, 4 provisionally and for 2 applications no information was available [4]. Figure 1 summarizes the current status of the applications under review and decided in a clear manner.
DiGAs in the European context
In addition to Germany, DiGAs are already reimbursable in France and Belgium. In Belgium, a three-stage approval process has been in place since April 2022, in order to subsequently receive reimbursement from health insurance funds [6]. Furthermore, in May 2023, France introduced a fast-track procedure called PECAN, which is similar to the German DiGA approval in many aspects [7]. In addition to France and Belgium, Austria has also been planning to introduce a DiGA system since mid-2023 (keyword here: E-Health Strategy 2024-2030) [8]. The aim is to introduce and integrate it into healthcare by 2026.
DiGA in the context of integrated care – a model for success or questionable benefit?
After 6 years with DiGAs, the question naturally arises as to whether these digital aids have noticeably improved medical care. From the perspective of representatives of the GKV-Spitzenverband (the central association of statutory health insurance funds), the German Association of Psychotherapists (DPtV), the Association of Family Doctors, and consumer advice centers, a "question mark" should rather be placed behind this point. In guest articles in an article by the AOK (a German health insurance company) from 2024, these parties are rather critical of DiGAs. The following criticisms were raised [9]:
- In reality, DiGAs tend to fall short of high expectations and thus do not noticeably improve healthcare.
- Manufacturers charge disproportionately high prices, while the benefit of some provisionally listed DiGAs is unclear.
- Requirements for data protection and product quality are not always sufficient.
- Therapy-replacing functions or exaggerated promises undermine the supportive idea of the actual conservative therapy and are thus associated with risks for patient safety.
- DiGAs are rather unfamiliar to prescribing physicians and psychotherapists and are therefore prescribed less frequently, as the effectiveness has not yet been clearly demonstrated in some areas (in the case of provisional listing) and further research is therefore necessary.
- Shift / devaluation of proven evidence criteria for prescribing a DiGA, which would have to be met before inclusion in standard care.
- Lack of information regarding the application spectrum, benefits, and effectiveness among prescribers and patients.
Associations and interest groups are therefore rather critical of DiGAs due to the high costs and questionable benefits. The legal framework for DiGAs should also be improved to clearly focus on the therapeutic benefit. At the same time, it is recognized that DiGAs offer the potential to improve medical care and to better network the various care services [10].
But is this criticism justified? Of course, some of the statements made by the relevant representatives must be seen in a certain context, e.g., that statutory health insurance companies and prescribers will never be happy about high costs for these. It is correct that there is a significant reduction in the initially called-up price, especially between the price of the DiGA application at initial listing and the actual price after negotiations between the manufacturers and the statutory health insurance companies. DiGA manufacturers are allowed to set a price within certain rules for the first 12 months in which the app is listed in the directory, but from the 13th month onwards, a price negotiated between health insurance companies and the manufacturer applies [11]. Therefore, one of the demands of the GKV-Spitzenverband is that prices should be negotiated between the GKV and the manufacturers from day one. This procedure is used, for example, for digital care applications (DiPA), which are only prescribed with confirmed medical benefits and have a reimbursement cap of €150 per quarter [12].
In comparison to the above-mentioned associations or institutions, the Spitzenverband Digitale Gesundheitsversorgung e.V. (SVDGV), naturally, draws a more positive conclusion. The association sees the continuous and sustainable growth in the DiGA market as a positive development. This growth has a positive impact on investments, research and development, among other things. The association also sees the trial year as an important instrument compared to the GKV, in order to be able to offer a digital care service for a large number of clinical pictures.
In addition, it is emphasized that a positive care effect had to be demonstrated by means of a randomized-controlled clinical study for both a provisional and a permanent listing.
However, the SVDGV sees a need to catch up in making DiGAs known to patients, doctors and psychotherapists. The lack of awareness of this novel form of care would lead to them being prescribed less frequently, thus limiting accessibility for suitable patients. However, this would be a decisive factor in order to be able to exploit the future care potential [13].
And what is the reputation of DiGAs among those for whom they are centrally important: their users?
Answering this question is complex. Valid numbers from large cohorts are rare. For a contribution by the Fraunhofer Institute from the end of 2023, a comparison of satisfaction ratings was made in the Apple App Store for permanently / provisionally included DiGAs and the respective top 100 (by popularity - not rating ) of paid / free apps in the medical field. The average star rating of permanently included DiGAs was only 3.68 / 5, and 3.50 / 5 stars for temporarily included DiGAs. Meanwhile, the average for the top 100 applications is 4.10 / 5 stars.
Thus, there is a substantial difference between the most popular health apps in the App Store and the DiGA representatives. According to a contribution by the Fraunhofer Institute, this has to do with the fact that the texts of the top 100 apps tend to create a more positive association among users than those of the DiGAs (investigation using a BERT model).
The best-rated DiGAs particularly emphasized ease of use, the possibility of interaction (asking questions, receiving answers, advice, chat) and the information offered [12].
The DiGA Report 2022 of the Techniker Krankenkasse (a German health insurance company) was also mentioned. According to this, approximately 63% of those surveyed agreed with the statement that the DiGA (rather) helps them to alleviate their symptoms. In addition, the dissatisfied users were asked about the reasons for their rejection of the DiGA used. The most frequent reasons given were a lack of added value compared to other web and app applications (26.6%), a low level of individualization and orientation towards the specific problems of the users (24.5%) and a lack of user-friendliness (16.3%) [14].
In a survey by the Stiftung Gesundheit (a German health foundation), around 40% of doctors saw their limited familiarity with the digital innovation as a factor that negatively influenced their acceptance. In contrast, existing clinical evidence would tend to increase the acceptance of 66% of the doctors surveyed. The following problems were also mentioned: complicated DiGA activation process (34%), insufficient information material (32%) and a lack of opportunity to test an application (47%) [15].
In summary, it remains debatable in the future whether these acceptance or user satisfaction values can justify the high costs associated with DiGAs.
"Survival of the Fittest": Critical Success Factors
Once the milestone of listing for a DiGA has been reached, the aim is to become established in the DiGA directory. As we have already noted, provisional inclusion (as well as permanent listing) does not mean the end of the road. The product must now continue to prove itself in the clinical environment and gain acceptance among users. This also includes, for example, standing up to competing products that will gradually enter the market. Perhaps even the great success of the product will encourage someone to develop a comparable product. It is therefore important to consider critical success factors during development and during the listing process to ensure survival in the market.
A systematic review from 2024 summarized these factors, which have a significant impact on the marketability of DiGAs. Generally speaking, these included a patient-centered design, the effectiveness of the application itself, user-friendliness, and compliance with data protection and information security regulations through standardized approaches.
In the following, these success factors are discussed in more detail, each with overarching examples [16]:
- Technical success factors (Design and functionality that are user-friendly/centered and meet the (individual) needs of patients and healthcare professionals; consideration of data protection and security concerns to strengthen user trust and ensure data security and privacy; provision of technical support for end users; scalability and use of agile development methods; conducting thorough testing to ensure functionality and usability).
- Social success factors (Involving end users in the design process; taking into account the different cultures and age groups as well as the linguistic diversity among end users and developing a common language; training and onboarding of end users; proof of clinical effectiveness and consideration of social or patient-related benefits; understanding patient needs and preferences, including patient engagement, provider acceptance, and cultural norms in healthcare).
- Network Management (Ensuring that all stakeholders are involved in the development and implementation process; building strong networks and partnerships; effective communication and coordination between stakeholders).
- Organizational success factors (Structures and processes within healthcare organizations that facilitate adoption, such as resource allocation and workflow integration; offering continuous training, education and support for physicians and team members; integration into existing workflows, guidelines and organizational goals to ensure seamless adoption and use; aligning digital health services with existing care processes; flexibility and adaptability).
- Ethical success factors (Considering the protection of privacy and confidentiality and obtaining informed consent from end users, e.g. for data collection and use; ensuring the ethical use of patient data and respect for patient autonomy; addressing issues related to data ownership, management and deletion).
- Regulatory success factors (Compliance with relevant laws, regulations and standards for the collection, storage and use of data; knowledge of relevant regulations and close cooperation with legal experts or supervisory authorities).
By considering and implementing these critical success factors, you as a DiGA manufacturer can increase your chances of long-term success and make your product "market-proof".
The future of DiGAs
There is a lot in flux: for example , the application-accompanying success measurement (AbEM), which is expected to be mandatory from 2026, the interaction with the newly introduced electronic patient record, or the audit criteria for data protection for DiGA and DiPA via the BSI TR-03161 as a new basis for verification, to name just a few examples.
Aspects such as "gamification", the use of artificial intelligence, the embedding of therapeutic components of the DiGA as an app in platform models or the special data potential through the collected DiGA data in Europe can have a lasting positive influence on the use and acceptance of DiGAs. In the future, user feedback should also be better considered in development and further development. There is also potential at the regulatory level to promote DiGAs in the future (e.g. with regard to the digitalization strategy). For example, the uncertainty about the prescription process could be resolved by the e-prescription in the care system and DiGAs could be more strongly integrated into digital healthcare as a meaningful innovation [15].
Funding Opportunities
At this point, we would like to briefly discuss the funding opportunities for DiGAs. Small and medium-sized enterprises (SMEs) are to receive support through appropriate funding measures. An example here would be a possible BMBF funding measure for the "Clinical Validation of Innovative Medical Technology Solutions". The funded content includes the preparation and implementation of clinical trials. Only SMEs with an operating facility or branch in Germany are eligible to apply. The involvement of other actors in the sub-contract (CROs, hospitals, etc.) is also possible [17].
Conclusion
Digital health applications have become an increasingly integral part of regular healthcare in recent years. Steadily increasing admission numbers with an increasing number of permanent listings in the DiGA directory prove this. It seems that Germany's pioneering role with regard to DiGAs has rubbed off on our direct European neighbors such as France, Belgium and Austria.
But the digital aids themselves are still under discussion in our country, especially because of the pricing and sometimes excessive expectations of the apps, which cannot be fulfilled. At the same time, however, it is recognized that DiGAs offer the potential to improve medical care and to better network the various care services. However, there is still room for improvement in the areas of acceptance and user satisfaction in order to be able to take the broad community of users and doctors with us. Critical success factors must be taken into account as a manufacturer in order to keep the product on the market for as long as possible. In the future, there will still be some hurdles for these technologies, including in the areas of regulation and data protection, although a great potential of these digital aids is seen through further development, combination with future technologies and as an excellent data source for medical research.
Sources
- DiGA Guide v. 3.5, 28.12.2023
- diga.bfarm.de/de (Accessed 17.03.25)
- www.bfarm.de/DE/Medizinprodukte/_FAQ/DigiG/_artikel.html (Accessed 12.03.25)
- https://diga.bfarm.de/de/verzeichnis (Accessed 18.03.25)
- www.bfarm.de/DE/Medizinprodukte/Aufgaben/DiGA-und-DiPA/DiGA/Wissenswertes/_artikel.html, (Accessed 18.03.25)
- www.dsv-europa.de/de/news/2022/04/belgien-erstattet-digitale-gesundheitsanwendung-diga.html (Accessed 12.03.25)
- www.handelsblatt.com/inside/digital_health/gesundheitsapps-neuer-markt-fuer-deutsche-firmen-wie-attraktiv-ist-die-franzoesische-diga/29136588.html (Accessed 12.03.25)
- www.sozialministerium.at/dam/jcr:6f5c5706-b2c4-48a2-8b6a-c7f72f9580e3/240806-eHealth-bf.pdf (Accessed 12.03.25)
- www.aok.de/pp/gg/magazine/gesundheit-gesellschaft-09-2024/rundruf-diga/ (Accessed 17.03.25)
- www.gkv-spitzenverband.de/gkv_spitzenverband/presse/fokus/fokus_diga.jsp (Accessed 17.03.25)
- Schmidt L et al. The three-year evolution of Germany‘s Digital Therapeutics reimbursement program and its path forward. NPJ Digit Med. 2024 May 24;7(1):139.
- www.iese.fraunhofer.de/blog/digitale-gesundheitsanwendungen-zwischenfazit/ (Accessed 17.03.25)
- www.digitalversorgt.de/wp-content/uploads/2024/01/DiGA-Report-2023-SVDGV.pdf (Accessed 17.03.25)
- www.tk.de/resource/blob/2125136/dd3d3dbafcfaef0984dcf8576b1d7713/tk-diga-report-2022-data.pdf (Accessed 17.03.25)
- Schlieter H et al. Digitale Gesundheitsanwendungen (DiGA) im Spannungsfeld von Fortschritt und Kritik : Diskussionsbeitrag der Fachgruppe „Digital Health“ der Gesellschaft für Informatik e. V. Bundesgesundheitsbl . 2024 Jan;67(1):107-114. German.
- Schramm L et al. Critical success factors for creating sustainable digital health applications: A systematic review of the German case. Digit Health. 2024 Apr 24; 10:20552076241249604.
- www.projekt-portal-vditz.de/bekanntmachung/klinische-validierung-innovativer-medizintechnischer-loesungen (Accessed 18.03.25)

