What is actually the difference? This is an often discussed question for which there is no universal answer.
While the MDR may only use the term (other) clinical investigation, according to ISO 14155, the term clinical investigation is equivalent to "clinical trial" or "clinical study".
Whether registration study, post-market surveillance, registry study, user questionnaire or other clinical trials: for simplification purposes we use the term "clinical study" across the board.
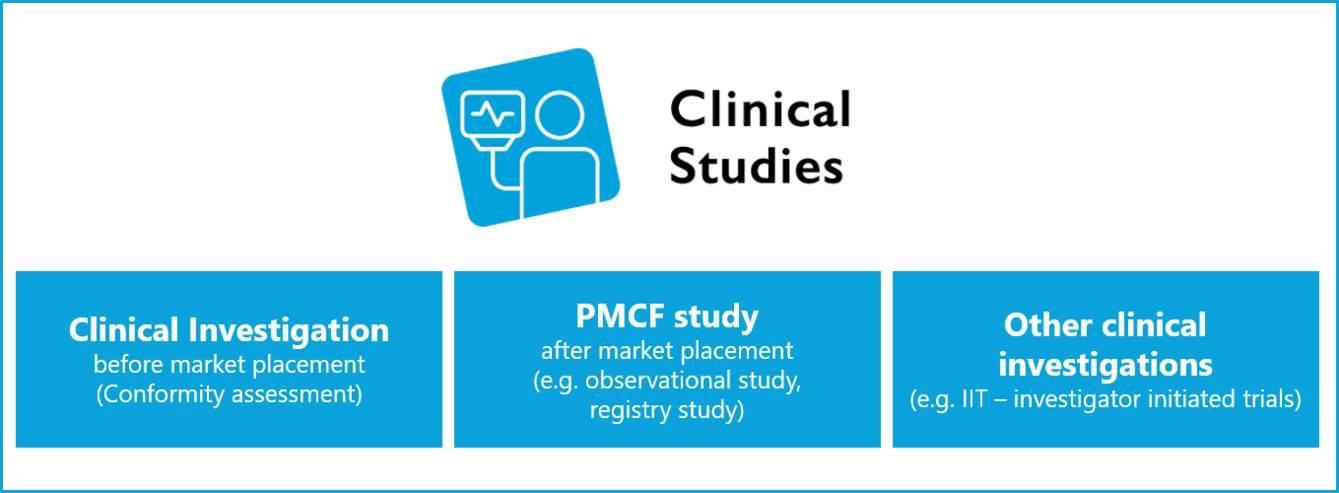
According to MPDG, article 1, § 3, other clinical investigations
- are not part of a planned and systematic process for device development or device observation of a current or future manufacturer
- are not carried out with the aim of demonstrating the conformity of a device with the requirements of Regulation (EU) 2017/745
- serve to answer scientific or other questions and
- are beyond the scope of a clinical development plan in accordance with Annex XIV, Part A, Point 1(a) of Regulation (EU) 2017/745.
Contact
We provide tailored support to meet your needs, whether it is a clinical study for a declaration of conformity of a medical device or a PMCF measure in as a part of post-market surveillance (PMS).
Email: studies@novineon.com
Phone: +49 7071 98979 - 139

